Electrolyzed Oxidizing (EO) water
EO water is produced by passing a diluted salt solution through an electrolytic cell consisting of an anode and a cathode. The two electrodes will be separated by a membrane and connected to direct current voltages, which results in accumulation of negatively charged ions such as chloride and hydroxide in the anode side to give up electrons. This results in generation of oxygen gas, chlorine gas and hypochlorite ion, hypochlorous acid and hydrochloric acid. The positively charged ions such as hydrogen, sodium move to the cathode to take up electrons and become hydrogen gas and sodium hydroxide. In this process two types of water are produced simultaneously as shown in the picture.

- Electrolyzed acidic water or Electrolyzed oxidizing (EO) waterProduced from anode side
pH: 2.3 to 2.7
ORP: > 1000 mV
High concentrations of dissolved O2 and free chlorine - Electrolyzed alkaline water or Electrolyzed reduced (ER) waterProduced from cathode side
pH: >11
ORP: -800 to -900 mV
High concentrations of dissolved NaOH and H2
Bactericidal mechanism: Bacteria generally grow in a pH range of 4-9, ORP range +200 to 800 mV (aerobic bacteria) and -700 to +200 mV (anaerobic bacteria). Low pH of EO water sensitize the outer membrane of bacterial cells to the entry of HOCl into bacterial cell. HOCl is the most active of chlorine compounds and it is reported to kill bacteria through inhibiting glucose oxidation by chlorine oxidizing sulfhydryl groups of certain enzymes important in carbohydrate metabolism. In addition, chlorine compounds reported to disrupt protein synthesis, oxidative decarboxylation of aminoacids to nitrites and aldehydes, reactions with nucleic acids, purines and pyramidines, destruction of DNA transforming ability, inhibition of oxygen uptake, formation of toxic N-chlorine derivatives and creation of chromosomal aberrations. High ORP of EO water believed to result in cellular damage through oxidation, disruption of metabolic processes and essentially killing the cell.
Levulinic Acid and Sodium Dodecyl Sulfate (LA-SDS)
Levulinic acid plus sodium dodecyl sulfate (LA-SDS) solution is proven to be very effective against foodborne pathogens on produce and poultry. Its efficacy against bacterial pathogens in the presence of organic debris makes it an attractive alternative to chlorine based sanitizers. Levulinic acid and SDS are generally recognized as safe by the U.S. Food and Drug Administartion (FDA) for use as food additives. LA can be used as a direct food additive for flavoring while SDS is approved as a multipurpose additive for food and non-food applications and has been widely studied as a surfactant. During the past 15 years at the University of Georgia, a wide array of LA and SDS either individually or in combination have been proposed for practical and effective treatment to kill foodborne pathogens including E. coli O157:H7, Salmonella, Listeria monocytogenes, and Campylobacter on foods. They found neither levulinic acid nor SDS alone provided a substantial reduction in killing E. coli O157:H7 or S.Enteritidis. However, a combination of 0.5% LA and 0.05% SDS resulted in ca. 7 log CFU/ml reduction of E. coli O157:H7, S. Enteritidis and S. Typhimurium DT104 within 1 min of exposure.
Peracetic acid (PAA)
Peracetic acid (PAA) is a sanitizer approved by U.S. Food and Drug Administration for application on food contact surfaces and direct application on fruits, vegetables, meat, poultry and seafood. PAA blend is formed in an equilibrium mixture of acetic acid and hydrogen peroxide. The ratio of acetic acid and hydrogen peroxide in PAA is unaffected by change in temperature, keeping the microbicidal efficacy of the PAA stable at a wide range of temperature. Although proportion of each component in the mixture can vary based on manufacturer, PAA acts on cell membrane lipoproteins and outer membrane lipoproteins of bacterial cell, enabling its antimicrobial activity against Gram-negative bacteria. It is water soluble and it decomposes into non-toxic by-products (e.g., oxygen and acetic acid, carbonic anhydride and water) after application. In meat processing industry, chlorine based sanitizer can react with protein generating byproducts which may be carcinogens. However, PAA based intervention treatments does not suffer from this drawback and is widely accepted all around world.
Germicidal UV Radiation (UVC)
Ultra-violet light is a form of electromagnetic radiation that falls between the wavelengths of 10 nm to 400 nm. UV radiation is situated between visible light and X-rays on the spectrum. The UV light region is further divided into many subdivisions but UV-A, UV-B and UV-C are frequently used. UV-C region ranges from 100-280 nm and has bactericidal properties. The bactericidal properties of UV-C are intense at a wavelength of 254 nm.
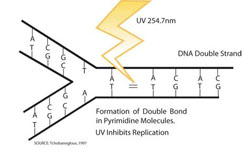
UV radiation is an important non-thermal disinfection system. The primary mode of action of UV light is to deactivate DNA. UV light when absorbed by DNA of the microbes, results in formation of photoproducts. Primarily UV light induces thymine-thymine dimers which will stop the DNA replication. The unzipping process of DNA strand is stopped when it reaches the thymine-thymine dimer and hence replication of DNA is suspended. This can ultimately lead to cell death.
Infrared Radiation
Infrared radiation ranges from 700 nm to 1 mm. Infrared radiation is further divided into three different regions depending on the wavelength as NIR (near infrared), MIR (Mid Infrared) and FIR (Far infrared). Shorter wavelength of IR has more penetration and less heat while longer length has less penetration with more heat. Absorption of IR energy by water or any organic matter is high at FIR; hence it is used for pasteurization. IR is widely used for many food processing operations such as drying, cooking and baking. Currently, application of IR for food surface sanitization is of growing interest.
The mechanism of IR radiation in killing bacteria is not clear. Researchers have cited RNA, DNA, ribosomes, cell envelope and cell proteins as some possible sites of inactivation that lead to bacterial death.
UV Activated Titanium Dioxide (UV-TiO2) Coating
Semiconductors (such as TiO2, ZnO, Fe2O3, CdS, and ZnS) can act as sensitizers for light-induced redox reactions due to their unique electronic structure, which is characterized by a filled valence band (VB) and an empty conduction band (CB).

As shown in the picture, when a photon with energy of hν matches or exceeds the band gap energy (Eg,) of the semiconductor, an electron (ecb-), is promoted from the VB into the CB, leaving a hole (hvb+) behind. Excited state CB electrons and VB holes can recombine and dissipate the input energy as heat, get trapped in metastable surface states or react with electron donars and electron acceptors adsorbed on the semiconductor surface from the surrounding environment. This phenomenon can be effectively harnessed for environmental safety of food systems.
The unique electronic band structure of TiO2 makes it as a stand-alone photocatalyst, and an ideal choice for the photocatalysis. TiO2 adsorbs photons with sufficient energy (λ< 380 nm or UVA) and results in generation of electrons (e-) – hole (h+) pairs. These conduction band electrons and valence band holes on the surface can react with surface bound O2 or H2O to initiate redox reactions so as to generate reactive oxygen species (ROS) like hydroxide radicals (OH-), superoxide radicals (O2-) and hydrogen peroxide (H2O2) in various chain reactions. The photo generated reactive oxygen species on the TiO2 coated surfaces can able to avoid surface cross-contamination by effectively killing pathogenic microorganisms as shown below.

Pulsed Light
Pulsed light (PL) is a technique for killing microorganisms, which usages short pulses of intense broad spectrum light, rich in UV-C. This technique is also known as “pulsed UV light”, “high intensity broad-spectrum pulsed light” and “pulsed white light”. Pulsed light is generated using a technology that increases the power manifold. During the process, power is magnified by storing electricity in a capacitor and then releasing the stored energy as light flashes in shorter time (millionths or thousandths of a second). The light flashes generated in PL consist of wavelengths ranging from 200 to 1100 nm. The UV-C component (280 nm – 200 nm) of pulsed light is responsible for the microbicidal properties of the PL. The microbicidal effect of PL is primarily due to the formation of thymine dimers. The dimer formation inhibits bacterial DNA replication, leading to cell death and bacterial inactivation. Furthermore, PL can also cause localized heating of bacterial cells leading to membrane destruction and disturbance of bacterial structure due to high intensity pulses. Gram-negative bacteria are more susceptible to PL than Gram-positive bacteria. The major advantage of this technique is high dosage of UV can be applied in very short time duration, resulting in very high efficacy. Thus, making it more suitable for situations where rapid disinfection is required. Pulsed light also limit oxidation reactions of food products, as only a short treatment time is required to deliver the same energy and regular UV treatment. Additionally, unlike chemical sanitizers, PL treatment neither has any chemical cost nor it leave any residual compounds making it more economical and environment friendly.
Radio frequency technology
Radio-frequency (RF) is an innovative heating technology that is highly effective in reducing microbial contamination and improving food quality. It has been used in the food processing industry for many decades for different processing applications including it’s potential for pathogen inactivation. RF heating technology may pasteurize and sterilize foods in industrial applications and achieved sufficient microbial inactivation. RF heating utilize electromagnetic energy at a frequency range of 1–300 MHz. that can be converted into heat in foods. The volumetric heating provides rapid heating rate for faster processing time with improved product quality. RF heating is similar to microwave (MW) heating, and is a result of application of electromagnetic waves to generate heat at regulated frequencies. The heat is generated within a product due to the friction generated by the molecular rotation of polar molecules within the product. Transfer of heat energy occurs by convention and conduction from hot medium to product in the conventional heating systems. In contrast to conventional heating, volumetric heating is observed in the RF heating systems by generation of heat inside the product due to the electromagnetic field. Volumetric heating might be defined as simultaneously heating inside and outside of the product approximately at the same heating rate. The effects of electromagnetic energy on food components are similar to those found using other methods of heating, although the more rapid heating results in shorter processing times and hence fewer changes to nutritional and sensory properties. Heating effects of RF include enzyme and protein denaturation, destruction of nucleic acids and other vital component as well as disruption of the cell membranes. RF has potential application for value added meat products allowing faster heating rates, reducing pre and post processing contamination and offering better quality. The advantage of this RF over other technologies is that RF is not a surface treatment therefore, it can penetrate deep on the meat and kill pathogens that have migrated to the inside.
